About the Lab
Hematopoietic and immune cells play a pivotal role in the support of host homeostasis. Their dysfunction underlies many pathologies, including autoimmune, infectious, hereditary, vascular, oncological, and other conditions.
The main interest of our laboratory is to gain insight into the mechanisms of hematopoietic development in steady-state and pathological conditions. We are specifically interested in intercellular interactions and intracellular molecular pathways that regulate inflammatory and antibacterial immune responses of innate immune cells, and in the development of cell therapy approaches based on the use of myeloid cells.
Our current studies continue and extend research that was undertaken in the laboratory when it was first founded in 1971 and headed by the famous Russian biologist N. G. Khrushchev.
Research fields
Main current research projects and topics:
— Developing a model to generate human macrophages from induced pluripotent stem cells;
— Developing gene-targeted approaches to regulate inflammatory and antibacterial responses of human macrophages;
— Deciphering the mechanisms underlying the generation and functional activity of myeloid-derived suppressor cells;
— Testing different conditions allowing the expansion and differentiation of hematopoietic stem cells (HSCs).
In our macrophage studies we use a model of macrophage differentiation from induced pluripotent stem cells (iPSCs) (Fig. 1, 2).
This allows to model tissue-residing macrophages and generate gene-edited macrophages (Fig. 2).
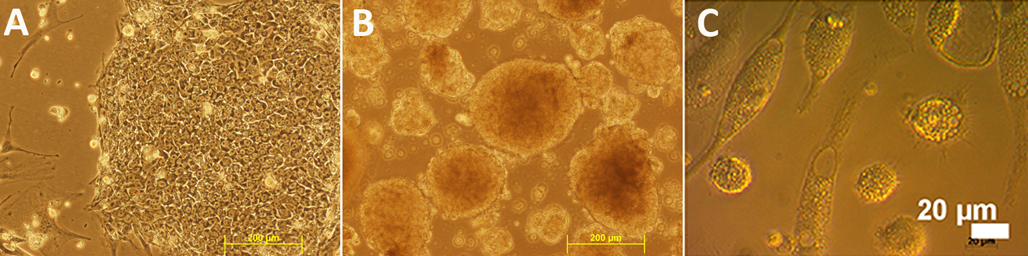
Figure 2. Main stages of macrophage differentiation from human iPSCs: iPSCs (А), Embryoid bodies (В), Macrophages (C).
We have recently demonstrated that iPSC-derived macrophages (iMphs) exhibit the typical macrophage morphology, express macrophage phenotype, and are highly phagocytic (Nenasheva et al., 2020). We are currently using this model to decipher the role of several specific genes in the regulation of macrophage protective and inflammatory responses. The long-term goal of the studies is to develop new therapeutic approaches to treat pathologies associated with macrophage dysfunction (Fig.3).

Figure 3. Main current and prospective iMph applications. Since the development of iMph generation techniques, the spectrum of iMphs applications has rapidly grown. Starting with the modeling of hereditary diseases associated with impaired macrophage function, it currently also includes the modeling of macrophage-pathogen interactions, drug testing and screening and the development of approaches to iMph-based cell therapy. BS Blau syndrome, CGD chronic granulomatous disease, CINCA chronic infantile neurologic cutaneous and articular syndrome; DENV Dengue virus, FMF familial Mediterranean fever, GD Gaucher disease, HTS high throughput screening, IBD inflammatory bowel disease, Mtb Mycobacterium tuberculosis, PAP pulmonary alveolar proteinosis, PD Parkinson’s disease, TD Tangier disease, ZIKV Zika virus.
From: Lyadova, I., Vasiliev, A. Macrophages derived from pluripotent stem cells: prospective applications and research gaps. Cell Biosci 12, 96 (2022). DOI.
Our second interest focuses on MDSCs, a heterogeneous population of immature myeloid cells able to regulate/suppress immune response (Fig. 1). MDSCs are implicated in the progression of many diseases, including cancer, infectious diseases, and autoimmune disorders, but the mechanisms regulating their generation and function, as well as their precise role in many pathologies are not fully understood. We previously reported the impact of MDSCs in the progression of tuberculosis infection in an experimental TB model in mice and humans (Lyadova et al., 2010; Tsiganov et al., 2014; Panteleev et al., 2017). Currently, we are planning to use in vitro and in vivo models to decipher the factors and mechanisms that determine MDSC generation and suppressor activity in steady-state conditions and in response to inflammation.
Hematopoietic stem cells represent an important source for the cell therapy of blood system diseases. Their wide usage is currently limited by the lack of compatible donors and the absence of methods for HSC expansion ex vivo. We are currently starting pilot experiments to test different methods of HSC expansion and differentiation in vitro (Fig.4).
The lab members

Irina Lyadova
M.D., PhD, Head of the Lab
Research Interests: the regulation of host antibacterial response and inflammation; hematopoiesis in steady-state and pathological conditions; infectious immunology; cell therapy.

Vsevolod Brodsky
PhD, Doctor of Biological Sciences, Professor, Honored Scientist of the Russian Federation, Chief Researcher.
Research Interests: cytology and histology, mechanisms and role of circadian (ultradian) rhythms in cell functions, mechanisms of polyploidization and properties of polyploid cells, analysis of intercellular interactions.

Nina Butorina
PhD, Senior Researcher.
Research Interests: the role of mesenchymal stromal cells (MSCs) in regeneration processes and the support of HSC quiescence/differentiations, HSC expansion and the potential for their differentiation into different cell types, biomaterials.

Olga Sheveleva
PhD, Junior Researcher.
Research Interests: cell biology, hematopoietic stem cells, extracellular vesicles, myeloid-derived suppressor cells (MDSCs), generation of MDSCs in vitro.

Daniil Antonov
Bachelor student, Laboratory assistant.
Research Interests: hematopoietic differentiation of immune cells, genetically modified immune cells, infectious diseases.

Dmitry Chevalier
Research Assistant, PhD student.
Research Interests: hematopoiesis, immunology, primary immunity.

Anna Dzebisashvili
Research Assistant.
Research Interests: immunology, molecular biology, hematopoiesis.

Olga Sakovnich
PhD, Research Engineer.
Research Interests: genetics, cell biology, aging.

Elena Protasova
Research Assistant, PhD student.
Research Interests: cell biology, induced pluripotent stem cells, macrophages.

Anastasia Mishukova
Graduate student.
Research Interests: iPSCs, embryoid bodies, hematopoietic cell differentiation, embryology.

Tatiana Tortunova
Laboratory Assistant.
Grants & Publications
Selected Publications
- Lyadova I., Vasiliev A. Macrophages derived from pluripotent stem cells: prospective applications and research gaps // Cell & Bioscience. – 2022. – Vol. 12. – Art. no 96. DOI: 10.1186/s13578-022-00824-4. – Q1.
- Klepikova A., Nenasheva T., Sheveleva O., Protasova E., Antonov D., Gainullina A., Chikina E., Sakovnich O., Gerasimova T., Nikitina I., Shevalie D., Lyadova I. iPSC-derived macrophages: the differentiation protocol affects cell immune characteristics and differentiation trajectories // Int. J. Mol. Sci. – 2022. – Vol. 23(24). – Art. no 16087. DOI: 10.3390/ijms232416087. – Q1.
- Sheveleva O. N., Lyadova I. V. Hemapoietic Stem Cell and Initial Stages of Hemopoiesis: Research Methods and Modern Concepts // Russian Journal of Developmental Biology. – 2022. – Vol. 53. – Р. – 389-404. – R.
- Brodsky V.Ya. Ultradian signals and direct cell-to-cell communication. Translation of the book published in Russian, Scientific World. – 2021. Online edition with changes. М.; Publishing Office Pero. – 2022. – 247 p.
- Brodsky V.Y. Gangliosides in Orchestration of Intercellular Communication, Development, Neuronal Pathology and Carcinogenesis // Russian Journal of Developmental Biology. – 2022. – Vol. 53. – Is. 1. – Р. – 15–26. DOI 10.1134/S1062360422010076. – Q4.
- Mashanov G., Nenasheva T., Mashanova A., Lape R., Birdsall N.J.M., Sivilotti L., Molloy J.E. Heterogeneity of cell membrane structure studied by single molecule tracking//Faraday Discussions. – 2021. DOI 10.1039/d1fd00035g. – Q2.
- Mashanov G.I., Nenasheva T.A., Mashanova T., Maclachlan C., Birdsall N.J.M., Molloy J.E. A method for imaging single molecules at the plasma membrane of live cells within tissue slices // Journal of General Physiology. – 2021. – Vol. 153. – Is. 1. DOI: 10.1085/jgp.202012657. – Q1.
- Lyadova I., Gerasimova T., Nenasheva T. Macrophages derived from human induced pluripotent stem cells: the diversity of protocols, future prospects, and outstanding questions // Frontiers in Cell and Developmental Biology. – 2021. – Vol. 9. – Art. no. 640703. DOI: 10.3389/fcell.2021.640703. – Q1.
- Nenasheva T., Gerasimova T., Serdyuk Y., Grigor'eva E., Kosmiadi G., Nikolaev A., Dashinimaev E., Lyadova I. Macrophages derived from human induced pluripotent stem cells are low-activated “naive-like” cells capable of restricting mycobacteria growth // Frontiers in Immunology. – 2020. – Vol. 11. – Art. №. 1016. DOI: 10.3389/fimmu.2020.01016. – Q1.
- Gilbert K., Hammond K.D., Brodsky V.Y., Lloyd D. An appreciation of the prescience of Don Gilbert (1930 – 2011): master of the theory and experimental unravelling of biochemical and cellular oscillatory dynamics // Cell Biology International. – 2020. – Vol. 44. – P. 1283 – 1298. DOI: 10.1002/cbin.11341. – Q3.
- Brodsky V.Ya. Cell-cell interaction disorders associated with senescence can be repaired // Russian Journal of Developmental Biology. – 2020 – Vol. 51. – Is. 4. – P. 261-266. DOI: 10.1134/S1062360420040025. – Q4.
- Brodsky V.Y., Zolotarev Y.A., Malchenko L.A., Andreeva L.A., Lazarev D.S., Butorina N.N., Kozik V.S., Myasoedov N.F. The administration of semax and HLDF-6 peptides to rats regulates protein synthesis rhythm in hepatocytes and corrects senescent disturbances // Russian Journal of Developmental Biology. – 2020. – Vol. 51. – Is. 2. – P. 99-105. DOI: 10.1134/S1062360420020034. – Q4.
- Butorina N.N., Payushina O.V., Sheveleva O.N., Novokreshchenova A.N., Domaratskaya E.I., Istranov L.P., Istranova E.V. Experimental study of the possibility of culturing of mesenchymal stromal cell and induction of osteogenic differentiation on collagen-based scaffolds of various modifications // Bulletin of Experimental Biology and Medicine. – 2020. – Vol. 169. – Is. 1. – P. 162-168. DOI: 10.1007/s10517-020-04843-4. – Q4.
- Lyadova I.V., Staricov A.A. COVID-19 and BCG vaccine: is there a link? // Russian Journal of Infection and Immunity. – 2020. – Vol. 10. – No. 3. – P. 459–468. DOI: 10.15789/2220-7619-CAB-1472. – Q.
- Sukhanov Yu.V., Vorotelyak E.A., Lyadova I.V., Vasiliev A.V. Mesenchymal Stem Cell therapy-is the vessel half full or half empty? // Russian Journal оf Developmental Biology. – 2020 – Vol. 51. – Is. 4. – P. 267-270. DOI: 10.1134/S1062360420040104. – Q4
- Sheveleva O.N., Payushina O.V., Butorina N.N., Domaratskaya E.I. The myogenic potential of mesenchymal stromal cells and their effect on skeletal muscle regeneration // Biology Bulletin. – 2020. – Vol. 47. – N 5. – P. 455-465. DOI: 10.1134/S106235902005009X. – Q4.
Library & Useful Links