About the Lab
The main topics of our laboratory are associated with the problems of evolutionary developmental biology. This approach is related to the study of different species development and the influence of various environmental factors on the ontogenetic processes.
An important area of laboratory research is a comparative analysis of the role of Hox genes in the specification of morphogenetic processes in vertebrates: fish and mammals, which differ in the number of Hox clusters and the features of morphogenetic regulation.
Our main area of research is a male reproductive system in Mammals, including testicular development, differentiation of somatic testicular cells, and in vitro spermatogenesis. We focus especially on studying a specific region of the testis which is called the rete testis. It is a system of interconnected cavities and tubules which connects seminiferous tubules with efferent ducts. The rete testis plays an important role in the testis physiology. Recently, we examined the rete testis development in fetal and postnatal mice and showed that cells of the rete testis epithelium could be grown in culture, and that they share some characteristics with Sertoli cells, which are the main supporting cells for germ cell development. Our data also suggest that a number of rete testis cells originate from Sertoli cells and can reinitiate the expression of Sertoli cell genes both in vivo and in vitro. We hope that rete testis cells may become a cell source for various biomedical applications.
RETE TESTIS DEVELOPMENT IN EMBRYOS
Formation of the rete testis during mouse embryonic development. // Developmental Dynamics. 2020; DOI: 10.1002/dvdy.242.
The rete testis became visible by SOX9 and PAX8 staining starting from E12.5. It was located in the mesonephros but connected with testis cords formed by Sertoli cells expressing SOX9, AMH, DMRT1. Between E13.5 and E14.5 (Fig. 1), AMH+ network of testis cords at the mesonephric side began to disintegrate in a gradient-dependent manner along the anterior-posterior axis of the gonad and connections between testis cords gradually lost AMH becoming a part of the rete. Cells combining features of Sertoli and rete cells (PAX8+ AMH+ and DMRT1+ AMH− cells) were detected starting from E14.5, suggesting that some rete cells originated from Sertoli cells. The rete ovarii, a female counterpart of the rete testis, developed in a similar way as the rete testis until E13.5.
Conclusions: A part of the rete testis originates from connections between testis cords. Evidence that Sertoli cells contribute to rete cells is provided.
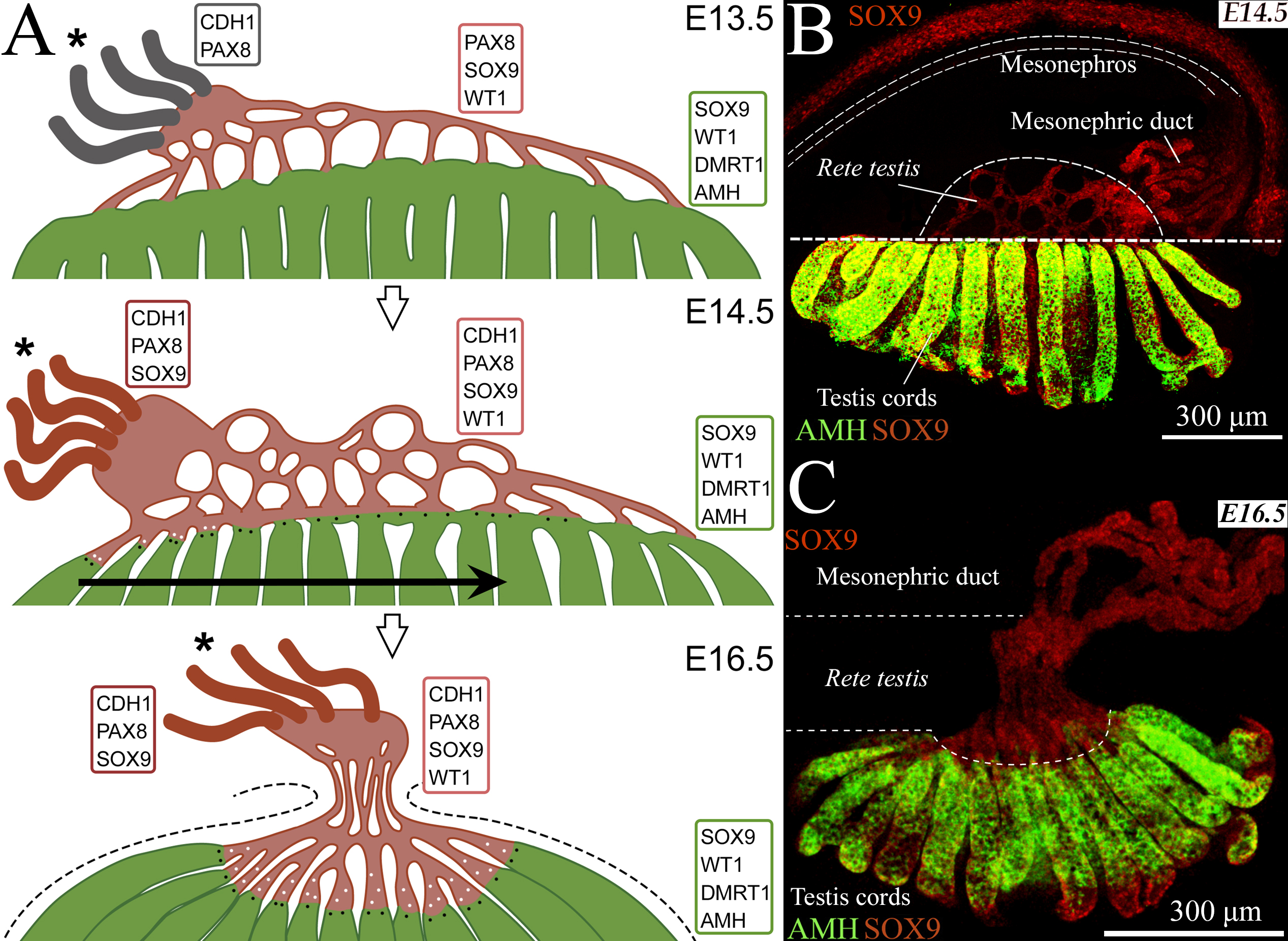
Figure 1. A- A diagram explaining the development of the rete testis between E13.5 and E16.5. Testis cords are depicted in green, rete testis structures are depicted in pale red. Mesonephric ducts are marked in grey at E13.5, when they are mostly SOX9 negative, and in bright red at E14.5 and E16.5, when they are SOX9 positive. Proteins expressing in each region are indicated. Asterisks: the anterior pole of the gonad; black dots: SOX9+ PAX8+ AMH+ cells; white dots: SOX9+ DMRT1+ AMH− cells. A horizontal arrow indicates the direction of the changes in the testis at E14.5. Dashed lines mark the testis boundaries at E16.5. B, C – Representative images of mesonephric gonad complex E14.5 (B) and E16.5 (C). Immunofluorescent detection of SOX9 and AMH.
Other studies:
The Rete Testis: Development and Role in Testis Function. // Russian Journal of Developmental Biology, 2021; DOI: 10.1134/S1062360421060072.
RETE TESTIS DEVELOPMENT AFTER BIRTH
The rete testis harbors Sertoli-like cells capable of expressing DMRT1. // Reproduction. 2019; DOI: 10.1530/REP-19-0183.
Sertoli cells (SCs) are supporting cells in the mammalian testis that proliferate throughout fetal and postnatal development but exit the cell cycle and differentiate at puberty. In our previous study, we isolated a population of highly proliferative Sertoli-like cells (SLCs) from the region of the adult mouse testis containing the rete testis and adjacent seminiferous tubules (DOI: 10.1530/REP-16-0013). Here RNA-seq of the adult SLC culture as well as qPCR analysis and immunofluorescence of the adult and immature (6 dpp) SLC cultures were performed that allowed us to identify SLC-specific genes, including Pax8, Cdh1, and Krt8 (Fig. 2). Using these, we found that SLCs are mostly localized in the rete testis epithelium; however, some contribution of transitional zones of seminiferous tubules could not be excluded. The main feature of SLCs indicating their relationship to SCs is DMRT1 expression. More than 40% of both adult and immature SLCs expressed DMRT1 at different levels in culture. Only rare DMRT1+ cells were detected in the adult rete testis, whereas more than 40% of cells were positively stained for DMRT1 in the immature rete testis. One more SC protein, AMH, was found in some rete cells of the immature testis. It was also demonstrated that SLCs expressed such SC genes as Nr5a1, Dhh, Gdnf, and Kitl and interacted with germ cells in 3D co-culture with immature testicular cells. All these similarities between SLCs and rete cells on one the hand and SCs on the other, suggest that rete cells could share a common origin with SCs.
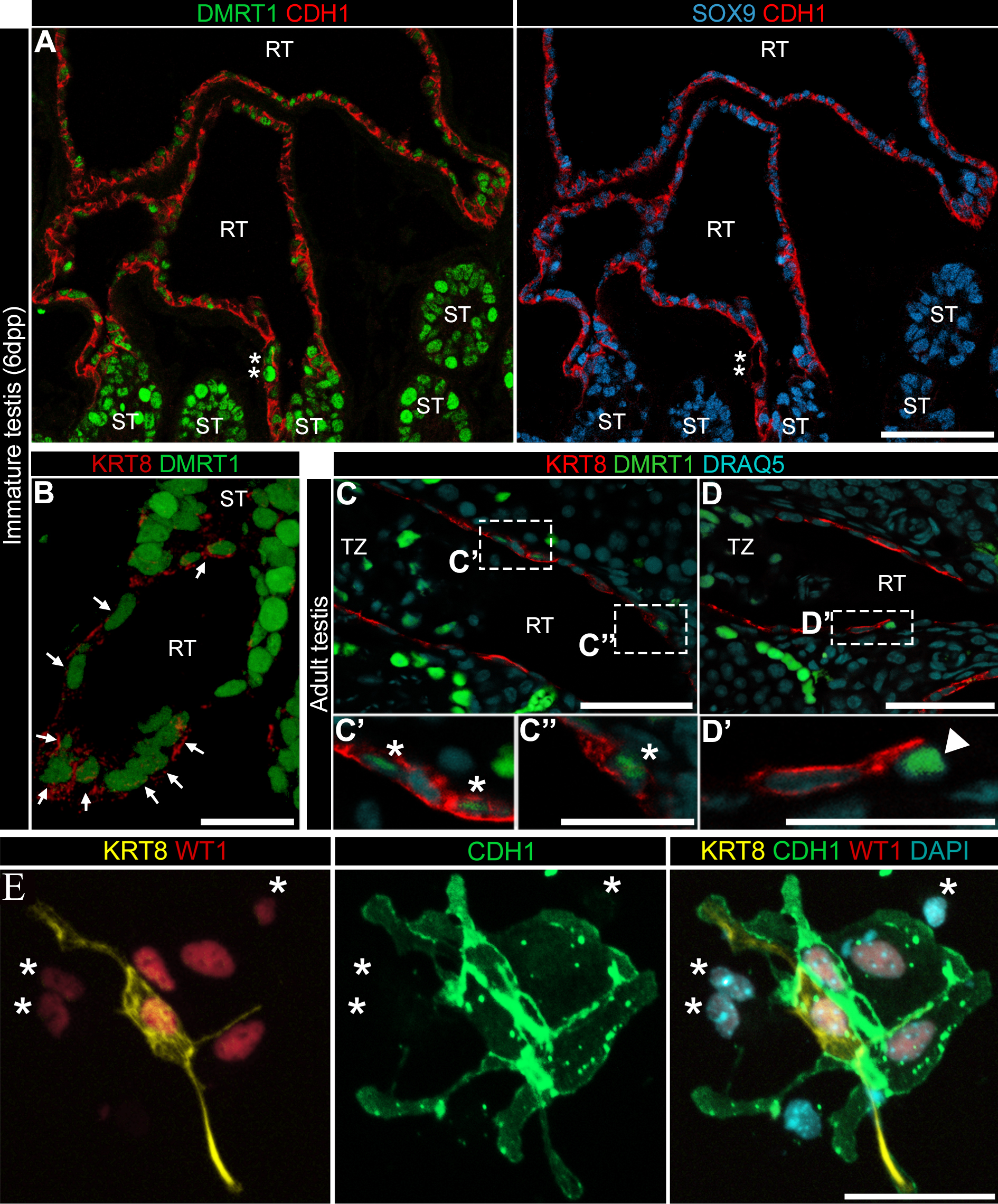
Figure 2. Cells in the immature (6 dpp – A, B) and adult (C, D) rete testis epithelium expressed DMRT1, CDH1 and SOX9. E - Immunofluorescence examination of adult SLCs at 1 day of culture. Clusters of SLCs, small WT1+ cells (red), were labeled with KRT8 (yellow) and CDH1 (green) antibodies. Asterisks indicate CDH1−KRT8− SLCs.
Other studies:
Rete testis and the adjacent seminiferous tubules during postembryonic development in mice. // Russian Journal of Developmental Biology. 2017; DOI: 10.1134/S1062360417060029.
Only a small population of adult Sertoli cells actively proliferates in culture. // Reproduction. 2016; DOI: 10.1530/REP-16-0013.
SERTOLI CELLS AND IN VITRO SPERMATOGENESIS
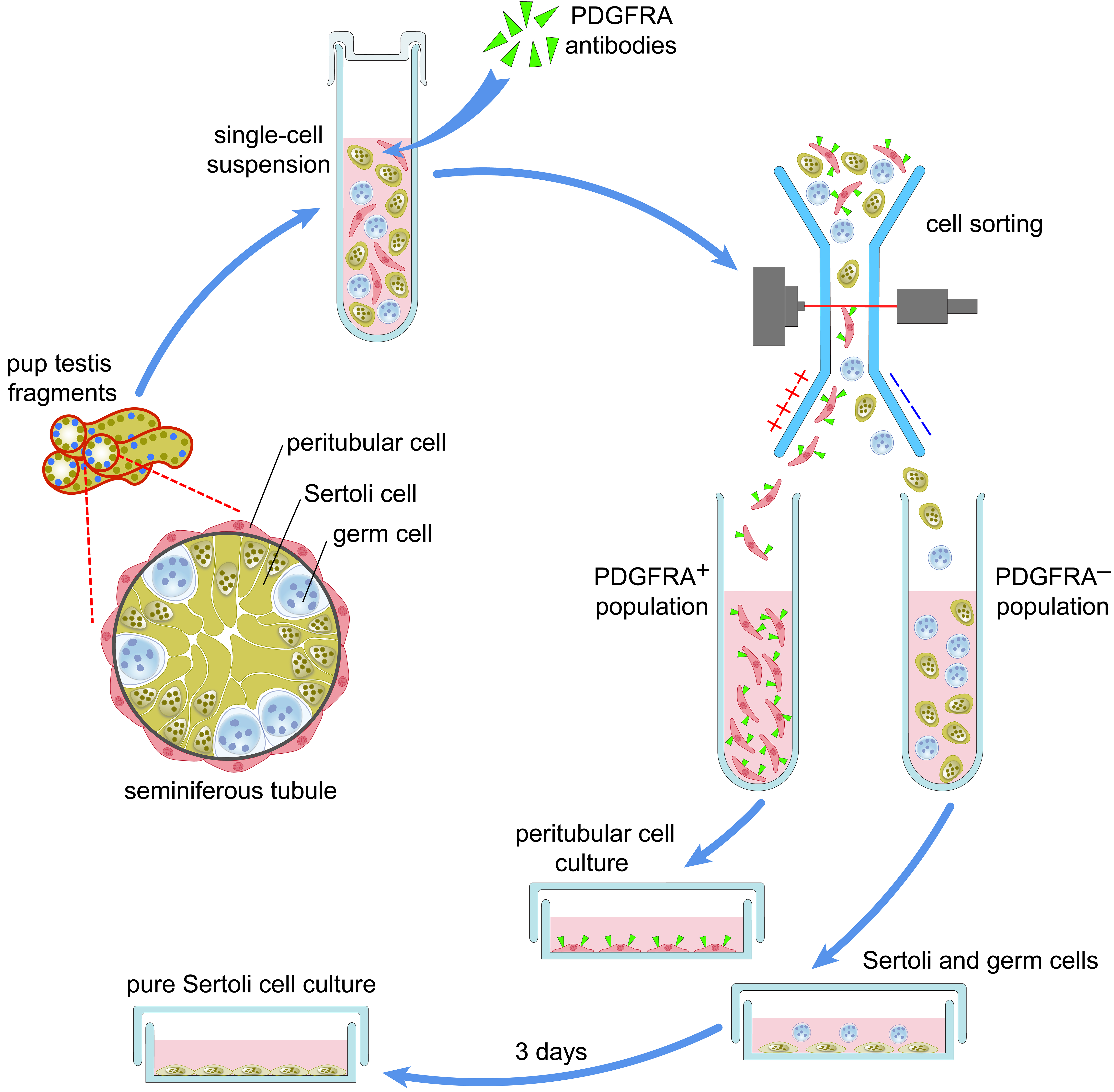
Establishment of a pure culture of immature Sertoli cells by PDGFRA staining and cell sorting. // Molecular Reproduction and Development. 2022; DOI: 10.1002/mrd.23574.
Sertoli cells are key somatic cells in the testis that form seminiferous tubules and support spermatogenesis. The isolation of pure Sertoli cells is important for their study. However, it is a difficult effort because of the close association of Sertoli cells with peritubular myoid cells surrounding seminiferous tubules. Here, we propose a novel approach to the establishment of a pure Sertoli cell culture from immature mouse testes (Fig. 3). It is based on the staining of testicular cells for platelet‐derived growth factor receptor alpha (PDGFRA), followed by fluorescence‐activated cell sorting and culturing of a PDGFRA‐negative cell population. Cells positive for a Sertoli cell marker WT1 accounted for more than 96% of cells in cultures from 6 to 12 days postpartum (dpp) mice. The numbers of peritubular myoid cells identified by ACTA2 staining did not exceed 4%. Cells in the cultures were also positive for Sertoli cell proteins SOX9 and DMRT1. Amh and Hsd17b3 expression decreased and Ar and Gata1 expression increased in 12 dpp cultures compared to 6 dpp cultures, which suggests that cultured Sertoli cells at least partially retained their differentiation status. This method can be employed in various applications including the analysis of differential gene expression and functional studies.
Other studies:
Neonatal testicular cell transplantation restores murine spermatogenesis damaged in the course of herpes simplex virus-induced orchitis. //Reproduction Fertility and Development. 2014. DOI: 10.1071/RD14255.
Genital tract infection and inflammation may affect male fertility, causing germ and Sertoli cell loss. We determined if testicular cell transplantation is effective at repairing testicular injury induced by herpes simplex virus (HSV) orchitis. ROSA26 mice were used as donors and the recipients were C57BL/6 mice after HSV testicular inoculation; some of the recipients were treated with the antiviral drug acyclovir (ACV). ACV reduced the amount of HSV antigen in testes on Day 3 after transplantation and enhanced the efficacy of transplantation at Day 30. In recipient testes, donor Sertoli cells formed new seminiferous tubules; significantly more new tubules were observed in the testes of ACV-treated mice compared with mice not treated with ACV(17.8% vs 3.6%, Fig. 4). Over half (50.4%) of new tubules in ACV treated testes contained germ cells and round spermatids were detected in 14.2% of new tubules compared with 15.9% and 5.3% in testes not treated with ACV, respectively. At Day 150 the seminiferous epithelium was completely recovered in some donor tubules and elongated spermatids were observed inside it.

Figure 4.. Transplantation of neonatal testicular cells into seminiferous tubules of ACV-treated recipient mice. (a, b) Minitubules and (c, d ) differentiating germ cells inside them are shown on Day 30 after transplantation; their blue staining (with X-gal) indicates that the minitubules are donor-derived. (e, f) X-Gal-positive (blue) minitubules and elongated spermatids (ES) inside them are shown on Day 150 after transplantation. (g) Magnified section of (b).SC, Sertoli cell; Sp, spermatocyte; RS, round spermatid. (a, b) Scale bars -100 μm. (c, d ) - 50 μm, (e, f) – 25 μm and g - 5μm.
Thus, our findings reveal the effectiveness of the combination of antiviral therapy with neonatal testis-cell transplantation for the restoration of spermatogenesis damaged by viral infection.
Other works:
In vitro spermatogenesis: In search of fully defined conditions. //Frontiers in Cell and Developmental Biology. 2023; DOI: 10.3389/fcell.2023.1106111.
The Behavior of Sertoli-Like Cells When Transplanted into Sertoli Cell-Depleted Testes. //Russian Journal of Developmental Biology. 2023; DOI: 10.1134/S1062360422060066.
Herpes simplex virus inoculation in murine rete testis results in irreversible testicular damage. // International Journal of Experimental Pathology. 2014; DOI: 10.1111/iep.12071.
Funding:
Russian Foundation for Basic Research, grant no. 16-34-60119. 2016-2018. “Studying the role of Dmrt1 in the differentiation and dedifferentiation of mouse Sertoli cells in postnatal development”.
Russian Science Foundation, grant no. 17-74-10076. 2017-2018. “Improvement of spermatogenesis in vitro technology: evaluation of the possibility to use adult testicular somatic cells for support of germ cell proliferation and differentiation”.
Presidium RAS Program “Fundamental research for biomedical technologies”, grant no. 0108-2018-0012. 2018-2019. “Improvement of a technique for correcting defects in spermatogenesis ex vivo using a population of adult Sertoli cells actively proliferating in culture.”
Russian Science Foundation, grant no. 23-24-00228. 2023-2024. “Investigation of the roles of Pax8 and Dax1 in the process of transdifferentiation of rete testis epithelial cells to Sertoli cells in the male mammalian gonad”.
Selected publications:
- Malolina E.A., Kulibin A.Yu., Naumenko V.A., …, Kushch A.A. Herpes simplex virus inoculation in murine rete testis results in irreversible testicular damage. // International Journal of Experimental Pathology. 2014. Vol. 95, №2. DOI: 10.1111/iep.12071.
- Kulibin A. Yu., Malolina E.A. Sertoli cells cultured under high-temperature and hypoxic conditions. // Cell and Tissue Biology. 2014. Vol.8, №2. DOI: 10.1134/S1990519X14020047.
- Naumenko V.A., Tyulenev Y.A., Kurilo L., Shileiko L., Sorokina T., Evdokimov V., Yakovleva V., Kovalyk V., Malolina E., Kulibin A., Gomberg M. and Kushch A. Detection and quantification of human herpes viruses types 4-6 in sperm samples of patients with fertility disorders and chronic inflammatory urogenital tract diseases. // Andrology. 2014. Vol. 2, №8. DOI: 10.1111/j.2047-2927.2014.00232.x.
- Malolina E.A., Kulibin A.Yu., Kushch A.A. Neonatal testicular cell transplantation restores murine spermatogenesis damaged in the course of herpes simplex virus-induced orchitis. // Reproduction Fertility and Development. 2014. Vol. 28, №6. DOI: 10.1071/RD14255.
- Kulibin A. Yu., Malolina E.A. Only a small population of adult Sertoli cells actively proliferates in culture. // Reproduction. 2016. Vol.152, №4. DOI: 10.1530/REP-16-0013.
- Malolina E.A., Kulibin A.Yu. Rete testis and the adjacent seminiferous tubules during postembryonic development in mice. // Russian Journal of Developmental Biology. 2017. Vol. 48, №6. DOI: 10.1134/S1062360417060029.
- Malolina E.A., Kulibin A.Yu. The rete testis harbors Sertoli-like cells capable of expressing DMRT1. // Reproduction. 2019. Vol. 158, №5. DOI: 10.1530/REP-19-0183.
- Kulibin A. Yu., Malolina E.A. Formation of the rete testis during mouse embryonic development. // Developmental Dynamics. 2020. Vol. 249, №12. DOI: 10.1002/dvdy.242.
- Kulibin A. Yu., Malolina E.A. The Rete Testis: Development and Role in Testis Function. // Russian Journal of Developmental Biology. 2021. Vol. 52, №6. DOI: 10.1134/S1062360421060072.
- Malolina E.A., Galiakberova A.A., Dashinimaev E.B., Kulibin, A.Y. Establishment of a pure culture of immature Sertoli cells by PDGFRA staining and cell sorting. // Molecular Reproduction and De-velopment. 2022. Vol.89, №5-6. DOI: 10.1002/mrd.23574.
- Kulibin A.Yu., Malolina E.A. In vitro spermatogenesis: In search of fully defined conditions. // Frontirs Cell Development Biology. 2023. V.11. DOI: 10.3389/fcell.2023.1106111.
- Mun. V.V., Kulibin A.Yu., Malolina E.A. The Behavior of Sertoli-Like Cells When Transplanted into Sertoli Cell-Depleted Testes. // Russian Journal of Developmental Biology. 2023. Vol. 53, №6. DOI: 10.1134/S1062360422060066.
The field of my research interests includes different aspects of the sea anemone species: population genetics and genomics, phylogenetics, reproduction, regeneration, evolution, and development. With my collaborators I found clonal and partially clonal populations of brooding sea anemones that can be used for further investigating the reproduction of the species and its adaptation to the environment. Also we investigate some morphological details of the nervous system of sea anemone early embryos. My additional project is the study of freshwater salmonid fishes in the Kamchatka Peninsula concerning the crucial role of abiotic factors for sympatric diversification as it is launched and maintained in the lacustrine and riverine environment.
One of the investigation areas is associated with the temperature influence on ontogenetic processes, in particular, on protein thermal stability. This parameter is studied for various proteins: serum albumin, tropomyosin, glycogen phosphorylase b, histone-like protein, and formiate dehydrogenase. For research in this area, we use the methods of differential scanning calorimetry and the high-resolution heteronuclear NMR spectroscopy.