About the Lab
Laboratory members:
Pavel Avdonin, Head of the Laboratory, Doctor of Biological Sciences, Professor
Marina Semenova, Senior Researcher, PhD
Petr Avdonin, Senior Researcher, PhD
Elena Rybakova, Researcher, PhD
Sergey Trufanov, Junior Researcher, PhD
Victor Podmarev, engineer
Research fields
We aim to study the role of receptors and cell signaling systems in the regulation of physiological processes. The following key results were obtained in the Lab.
- We discovered the role of lysosomal bipolar calcium channels in alpha1-adrenergic regulation of calcium ion exchange in smooth muscle cells of blood vessels and in vascular contractility [1].
- We described the functional role of 5-HT1B and 5-HT2B receptors in the regulation of calcium ion metabolism in endothelial cells [2].
- We demonstrated the involvement of NADPH oxidase in histaminergic regulation of Willebrand factor secretion by endothelium [3].
- We obtained evidence of Epac proteins participation in cAMP-dependent regulation of vascular contractility [4].
- Using the RNA interference method, we also showed that Orai-1 channels functioning in multinucleated cells of transverse striated muscle ensure the replenishment of calcium ions in sarcoplasmic reticulum [5].
- We have found that skeletal muscle cells have a system of InsP3-dependent regulation of calcium ion metabolism, which is formed during differentiation of myoblasts into multinucleated myotubules [6].

Fig. 1 Effect of 100 μM H2O2 and 1 U/ml thrombin on vWF exocytosis in HUVEC. Cell nuclei stained with Hoechst 33342 and vWF stained with ARC1779-Cy5 are shown in control cells (a) and after treatment with H2O2 (b) or thrombin (c). Elevation of vWF expression is shown in (d).

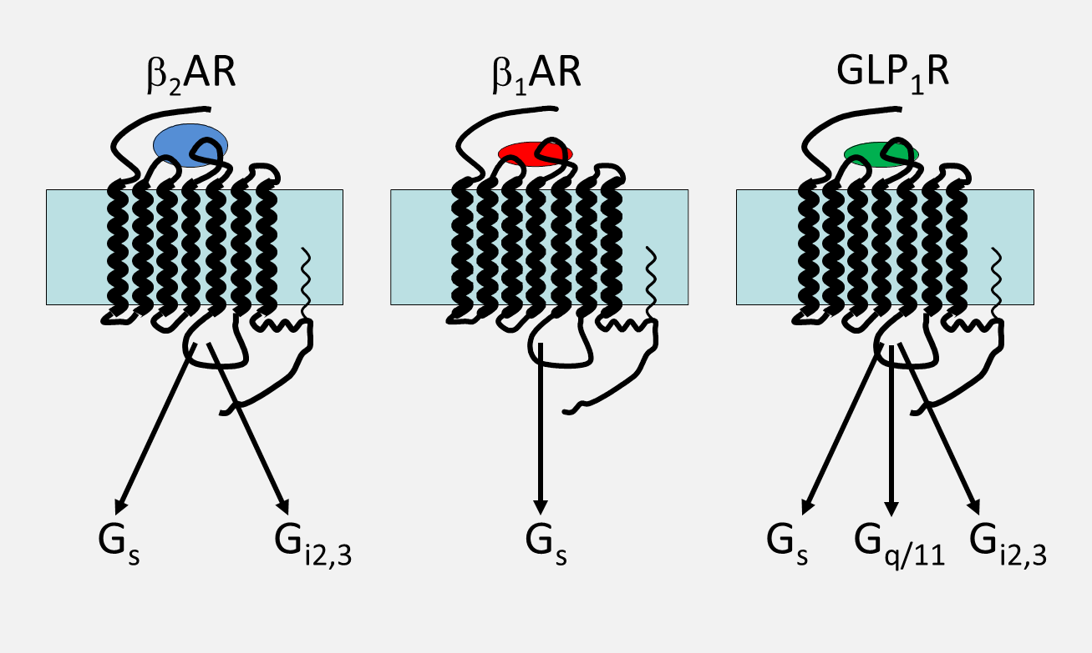
Fig. 2 Photoaffinity staining of G protein alpha-subunits in plasma membranes from mice heart after activation of β2-adrenoceptors. Abbreviations: C – control, Z – β2AR agonist zinterol, CCh – carbachol, I – isoproterenol, ICI – β2AR-antagonist, PTX – pertussis toxin.
The study of endothelial cell secretion and Willebrand factor exchange regulation is one of the main directions of the Laboratory's research work. We proposed a method of recording the Willebrand factor endocytosis using fluorescently stained aptamer that specifically binds to this protein on the surface of endothelial cells [7]. According to the data obtained, hydrogen peroxide can act as a secondary mediator and stimulate, similarly to calcium ions and cAMP, Willebrand factor exocytosis [7, 8] (Fig. 1). In collaboration with clinicians, we study Willebrand factor metabolism disorders in various forms of thrombotic microangiopathies in children [9-12] and adult patients [13-15].
In collaboration with Dr. S. Montrose-Rafizade (NIH, USA), we discovered new signaling pathways for β2-adrenoceptors through GTP-binding proteins Gi2,3 [16] (Fig.2,3) and conjunction of glucagon-like peptide 1 receptors with Gs, Gq/11 and Gi proteins [17] (Fig. 3). In the studies carried out together with Prof. Urs T. Ruegg (University of Geneva), we characterized the mechanisms of cyclosporin A regulation of vasopressin V1A-receptor expression [18] and angiotensin II AT1-receptor expression in vascular smooth muscle cells [19].
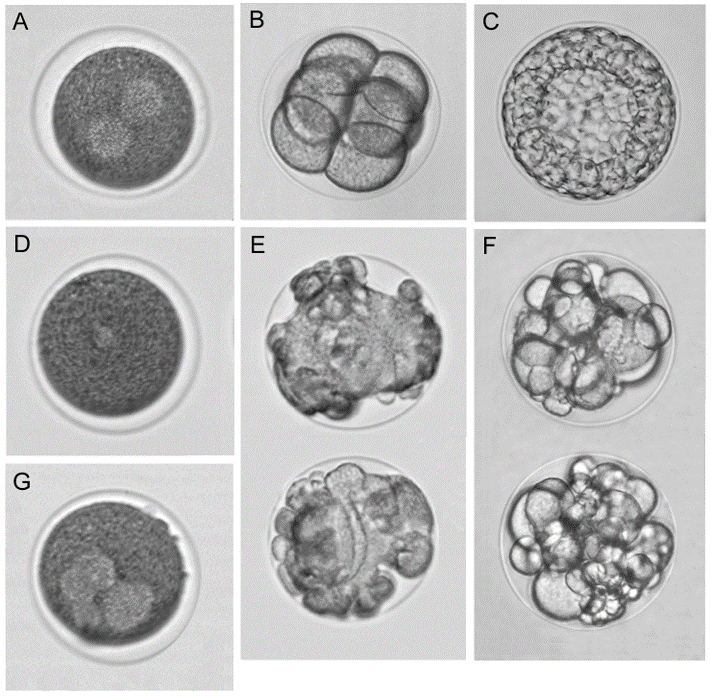
Fig. 4 Typical effects of tubulin/microtubule targeting compounds on the sea urchin Paracentr. otus lividus eggs and embryos. (A−C) Control: (A) Intact egg with normal bipolar mitotic spindle (light spots), the first cleavage anaphase; (B) eight-cell embryo; and (C) early blastula. (D−F) Effects of the microtubule destabilizer combretastatin A-4 at 20 (D and E) and 5 nM (F): (D) Arrested egg without mitotic apparatus; (E) tuberculate arrested eggs; and (F) cleavage alteration. (G) Aberrant multipolar mitotic apparatus in arrested egg in the presence of the microtubule stabilizer paclitaxel (5 μM). Compounds were added to zygotes at 8−15 min postfertilization. Samples were incubated at 21 °C. Eggs/embryos were observed at 1 h (A), 2.5 h (B, D, E, G), and 6 h (C, F) postfertilization. The average egg/embryo diameter is 115 μm. Ref: Semenova et al., ACS Comb Sci 2018.
Marina Semenova, a senior researcher of the Laboratory, is searching for substances with antimitotic activity using sea urchin embryos as a model. This model made it possible to identify dozens of substances of natural origin and their synthetic derivatives that block cell division by destabilizing microtubules (they can be viewed as potential antitumor substances) [20-26]. In collaboration with chemists from the RAS Institute of Organic Chemistry, Marina Semenova have designed a phenotypic sea urchin embryo assay for screening of chemical libraries for tubulin/microtubule targeting activity. The assay is highly reproducible yielding rapid information on antiproliferative, antimitotic, cytotoxic, and microtubule destabilizing effects of the molecules along with their solubility and permeability potential. Measured potencies of identified microtubule destabilizers correlated well with the values in both in vitro and cell-based conventional assays. The sea urchin embryo model provides reliable generation of 90-100 data points per day using standard light microscopy. To date, several hundred natural products and their synthetic derivatives suppressing cell division due to microtubule destabilization have been identified.
The hot topics of our investigations are a functional role of 5-HT1B and 5-HT2B receptors in endothelial cells, and interrelation of calcium ions and reactive oxygen species exchange in the vascular wall cells. In 2021, the Laboratory became involved in research work that aims to elucidate molecular and cellular mechanisms of thrombotic complications and complement system disorders in the patients with the coronavirus infection (COVID-19). We investigate the interactions of SARS-CoV-2 with vascular endothelial cells and blood cells leading to the complement system disorders and development of thrombotic complications in COVID-19.
Keywords: Receptors, ion channels, calcium ions, reactive oxygen species, blood vessels, endothelial and smooth muscle cells, Willebrand factor, complement system, COVID-19, thrombosis, screening, antimitotic activity, tubulin, sea urchin germ.
Grants
Grant No. 21-15-00441 of the Russian Science Foundation "Study of molecular and cellular mechanisms of thrombotic complications and complement system disorders in coronavirus infection (COVID-19). Searching for prognostic signs to assess the severity of COVID-19 course".
Selected publications & Bibliography
- Trufanov, S.K., et al., The Role of Two-Pore Channels in Norepinephrine-Induced [Ca(2+)]i Rise in Rat Aortic Smooth Muscle Cells and Aorta Contraction. Cells, 2019. 8(10).
- Avdonin, P.V., et al., Enhancement by Hydrogen Peroxide of Calcium Signals in Endothelial Cells Induced by 5-HT1B and 5-HT2B Receptor Agonists. Oxid Med Cell Longev, 2019. 2019: p. 1701478.
- Avdonin, P.V., et al., VAS2870 Inhibits Histamine-Induced Calcium Signaling and vWF Secretion in Human Umbilical Vein Endothelial Cells. Cells, 2019. 8(2).
- Sukhanova, I.F., et al., Activators of Epac proteins induce relaxation of isolated rat aorta. Dokl Biol Sci, 2006. 411: p. 441-4.
- Avdonin, P.V., et al., Expression and functional role of the protein Orai-1 in skeletal myoblasts and myotubes. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology, 2008. 2(4): p. 365-371.
- Muslikhov, E.R., et al., Elevation of the expression of the inositol-1,4,5-trisphosphate receptors and increase of calcium signaling in response to ATP during differentiation of skeletal myoblasts C2C12 into myotubes. Biochemistry (Moscow) Supplemental Series A: Membrane and Cell Biology, 2010. 4(4): p. 383-388.
- Avdonin, P.P., et al., The use of fluorescently labeled ARC1779 aptamer for assessimg the effect of H2O2 on von Willebrand factor exocytosis. Biochemistry (Mosc), 2021. 86(2): p. 1-9.
- Avdonin, P.V., et al., Hydrogen Peroxide Stimulates Exocytosis of Von Willebrand Factor in Human Umbilical Vein Endothelial Cells. Biology Bulletin, 2017. 44(5): p. 531-537.
- Orlova, O., et al., DEFICIENCY OF ADAMTS13 ACTIVITY IN STEC-HUS AND ATYPICAL HUS IN CHILDREN. Nephrology Dialysis Transplantation, 2018. 33: p. 307-307.
- Orlova, O., et al., THE ROLE OF ADAMTS13 DEFICIENCY IN SHIGATOXIN ASSOCIATED AND ATYPICAL HEMOLYTIC UREMIC SYNDROME IN CHILDREN. Pediatric Nephrology, 2017. 32(9): p. 1786-1786.
- Maschan, M., et al., Control of thrombotic thrombocytopenic purpura by sirolimus in a child with juvenile myelomonocytic leukemia and somatic N-RAS mutation. Pediatr Blood Cancer, 2014. 61(10): p. 1871-3.
- Maschan, A., et al., Bortezomib induces long-term remission in children with immune thrombotic thrombocytopenic purpura, refractory to plasma exchange, glucocorticoids, and rituximab: A report on two cases. Pediatr Blood Cancer, 2021. 68(4): p. e28818.
- Dzhumabaeva, B.T., et al., [A case of hemolytic-uremic syndrome with development of catastrophic antiphospholipid syndrome: diagnosis and clinical tactics]. Ter Arkh, 2010. 82(3): p. 56-60.
- Shostak, N.A., et al., Antiphospholipid syndrome in the structure of hematogenic thrombophilia in young and middle-aged patients with venous thrombosis. Ter Arkh, 2005. 77(5): p. 47-51.
- Stolyar, A.G., et al., [Clinical observation of a patient with thrombotic thrombocytopenic purpura with renal and intestinal lesions]. Ter Arkh, 2019. 91(7): p. 106-110.
- Xiao, R.-P., et al., Coupling of Я2-Adrenoceptor to Gi Proteins and Its Physiological Relevance in Murine Cardiac Myocytes. Circ Res, 1999. 84(1): p. 43-52.
- Montrose-Rafizadeh, C., et al., Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology, 1999. 140(3): p. 1132-40.
- Cottet-Maire, F., et al., Upregulation of vasopressin V1A receptor mRNA and protein in vascular smooth muscle cells following cyclosporin A treatment. Br J Pharmacol, 2001. 132(4): p. 909-17.
- Avdonin, P.V., et al., Cyclosporine A up-regulates angiotensin II receptors and calcium responses in human vascular smooth muscle cells. Kidney Int, 1999. 55(6): p. 2407-14.
- Demchuk, D.V., et al., Synthesis and antiproliferative activity of conformationally restricted 1,2,3-triazole analogues of combretastatins in the sea urchin embryo model and against human cancer cell lines. Bioorg Med Chem, 2014. 22(2): p. 738-55.
- Lichitsky, B.V., et al., Benzimidazolyl-pyrazolo[3,4-b]pyridinones, Selective Inhibitors of MOLT-4 Leukemia Cell Growth and Sea Urchin Embryo Spiculogenesis: Target Quest. ACS Comb Sci, 2019. 21(12): p. 805-816.
- Semenov, V.V., et al., Efficient Synthesis of Glaziovianin A Isoflavone Series from Dill and Parsley Extracts and Their in Vitro/in Vivo Antimitotic Activity. J Nat Prod, 2016. 79(5): p. 1429-38.
- Semenova, M.N., et al., Sea Urchin Embryo Model As a Reliable in Vivo Phenotypic Screen to Characterize Selective Antimitotic Molecules. Comparative evaluation of Combretapyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles as Tubulin-Binding Agents. ACS Comb Sci, 2018. 20(12): p. 700-721.
- Semenova, M.N., et al., Polyalkoxybenzenes from plants. 5. Parsley seed extract in synthesis of azapodophyllotoxins featuring strong tubulin destabilizing activity in the sea urchin embryo and cell culture assays. J Med Chem, 2011. 54(20): p. 7138-49.
- Stroylov, V.S., et al., Computational modeling and target synthesis of monomethoxy-substituted o-diphenylisoxazoles with unexpectedly high antimitotic microtubule destabilizing activity. Bioorg Med Chem Lett, 2020. 30(23): p. 127608.
- Tsyganov, D.V., et al., cis-Restricted 3-aminopyrazole analogues of combretastatins: synthesis from plant polyalkoxybenzenes and biological evaluation in the cytotoxicity and phenotypic sea urchin embryo assays. J Nat Prod, 2013. 76(8): p. 1485-91.