Research fields
The Laboratory of Eukaryotic Genomic Repeats (laboratory head Dr. Kalmykova) was established in 2005 at the Institute of Molecular Genetics of the Russian Academy of Sciences. In 2018, Dr. Kalmykova was awarded the Koltsov Prize for the series of works "The role of small RNAs in protection of genome integrity and telomere maintenance". In 2022, Dr. Kalmykova became the head of Laboratory of Developmental Epigenetics in IDB RAS that continues research of telomere biology and epigenetic mechanisms of transposon regulation. The key areas of interest of our laboratory are chromatin modification mechanisms and small RNA-mediated gene regulation – an epigenetic pathway that changes the activity of target genes without changing the DNA.
Epigenetics of telomeres
We are interested in the mechanisms regulating telomere stability during the development and aging, and use Drosophila melanogaster as a model organism. Telomeres not only protect the linear chromosomes ends, but also act as a complex regulatory system which is linked to a variety of cellular pathways controlling genetic stability. Telomeres participate in many important processes such as development, aging, and oncogenesis. Due to this fact many groups around the world are interested in the mechanisms of telomere homeostasis, which still remains poorly understood. Our team is studying evolutionary conserved components of the telomeric complex, which are necessary for the transcription regulation of telomeric repeats and maintenance of telomeric chromatin in the germline (Fig. 1).
Deep sequencing methods allow for genome-wide identification of targets of epigenetic factors. We often observe changes in the expression and chromatin structure not only of telomeric repeats, but also of regular “selfish” retrotransposons suggesting a similarity between telomere maintenance and anti-transposon defense mechanisms (Fig. 2).
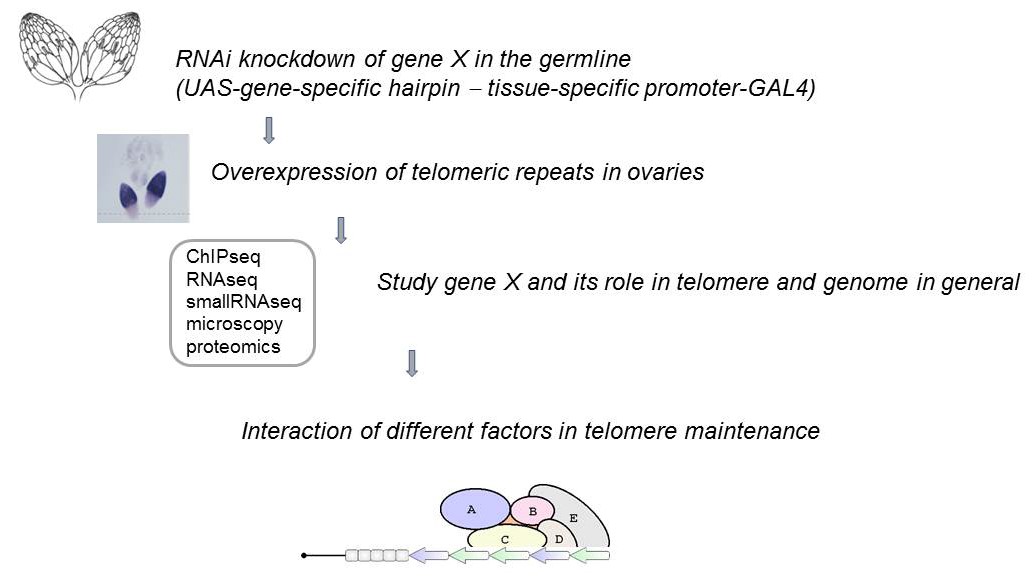
Fig. 1. Experimental strategy for the screening and studies of telomeric factors (Morgunova et al. 2015).
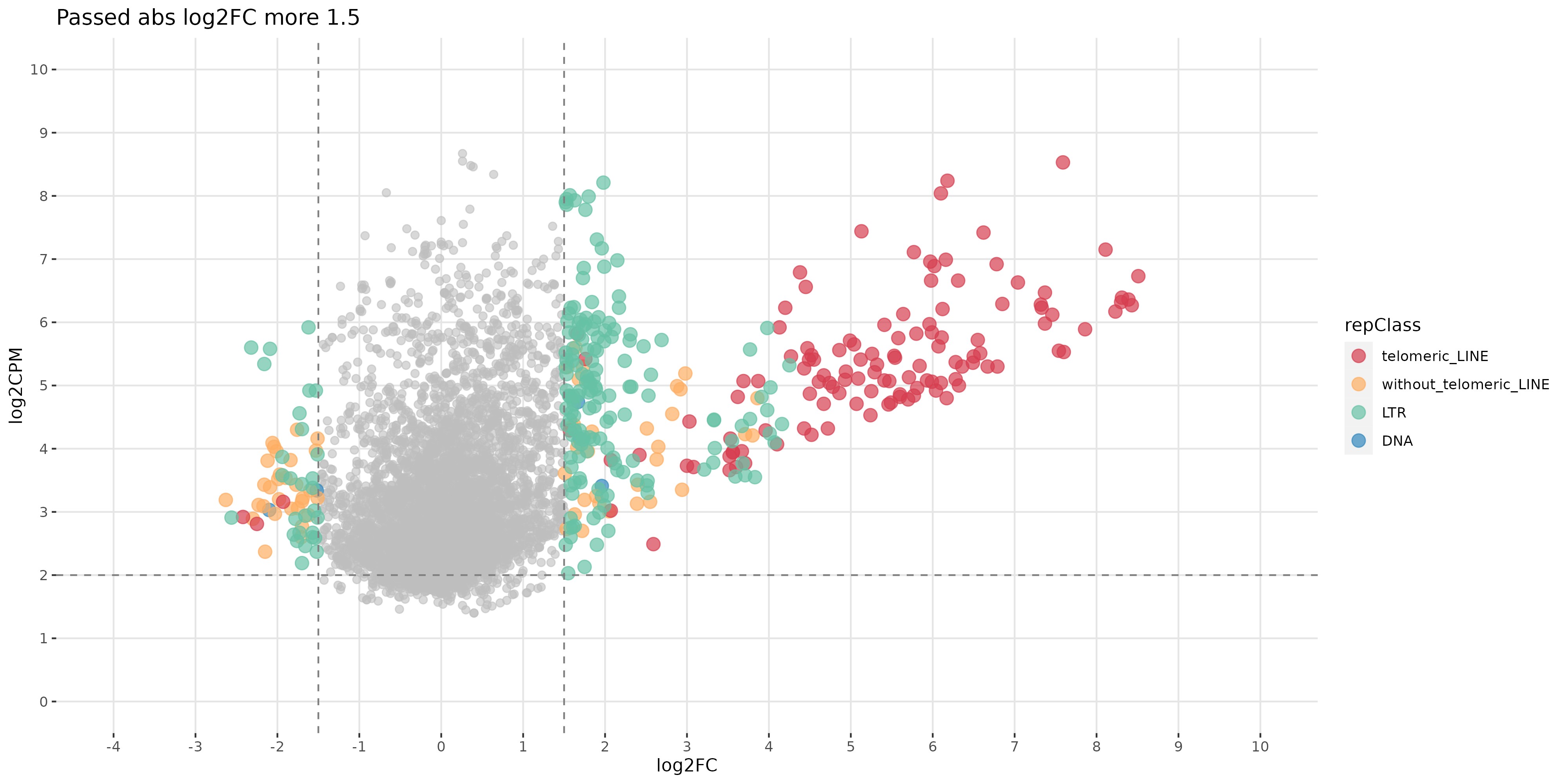
Fig. 2. Changes in the expression of telomeric repeats and other retrotransposons are observed after the germline knockdown of the RNA-binding protein Ars2, a conserved telomeric factor (unpublished data).
We have proposed, based on our research, the concept of telomeric checkpoint (Morgunova et al. 2021; Morgunova et al. 2023). According to this concept, telomeres serve as sensors that detect the signals of genome instability, and may trigger a response that leads to cell death or developmental arrest in case of telomere dysfunction. In the germline of lamin B mutants as well as old flies we observe an increased level of recombination in telomeres accompanied by telomeric DNA damage that leads to a loss of germ cells (Fig. 3). This leads to a conclusion that damage in the telomeric DNA may be a prime cause triggering cell death in both laminopathy and aging.
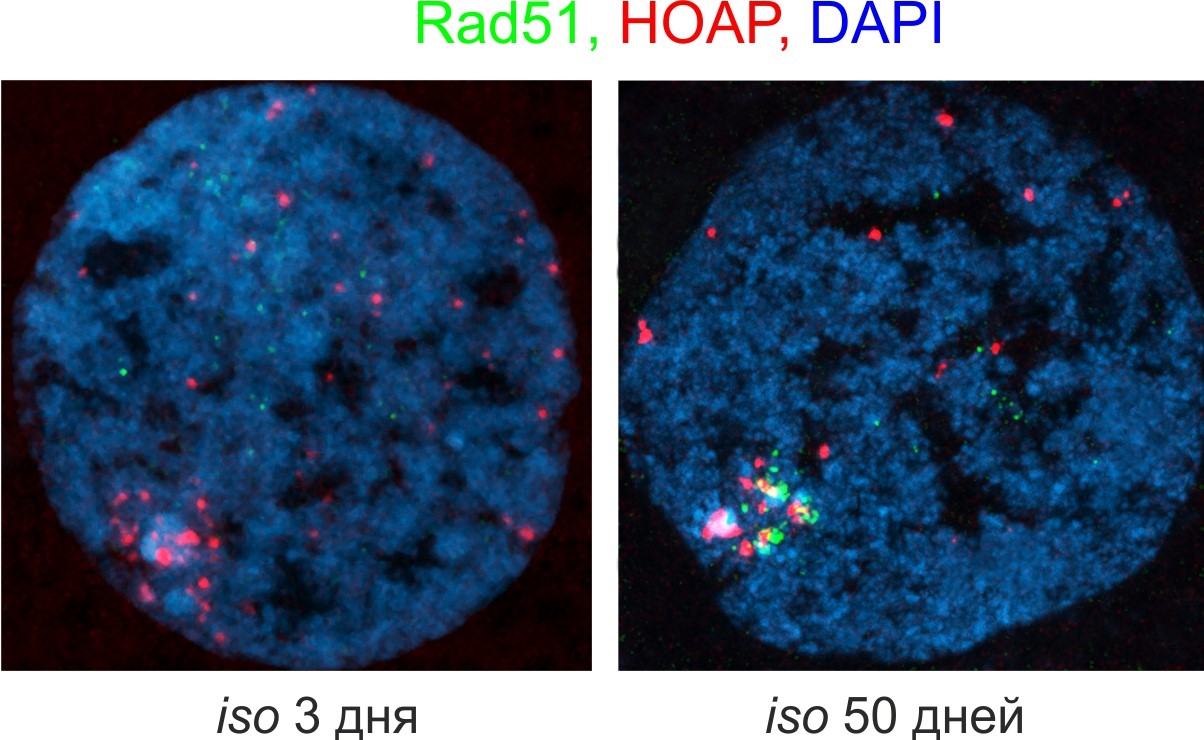
Fig. 3. Accumulation of recombinase Rad51 (green) in telomeres (telomeric protein HOAP, red) in the nucleus of aging Drosophila nurse cell. Here, the ovaries of 3- and 50-day-old flies of the isogenic strain iso-1 were studied (Morgunova et al. 2022).
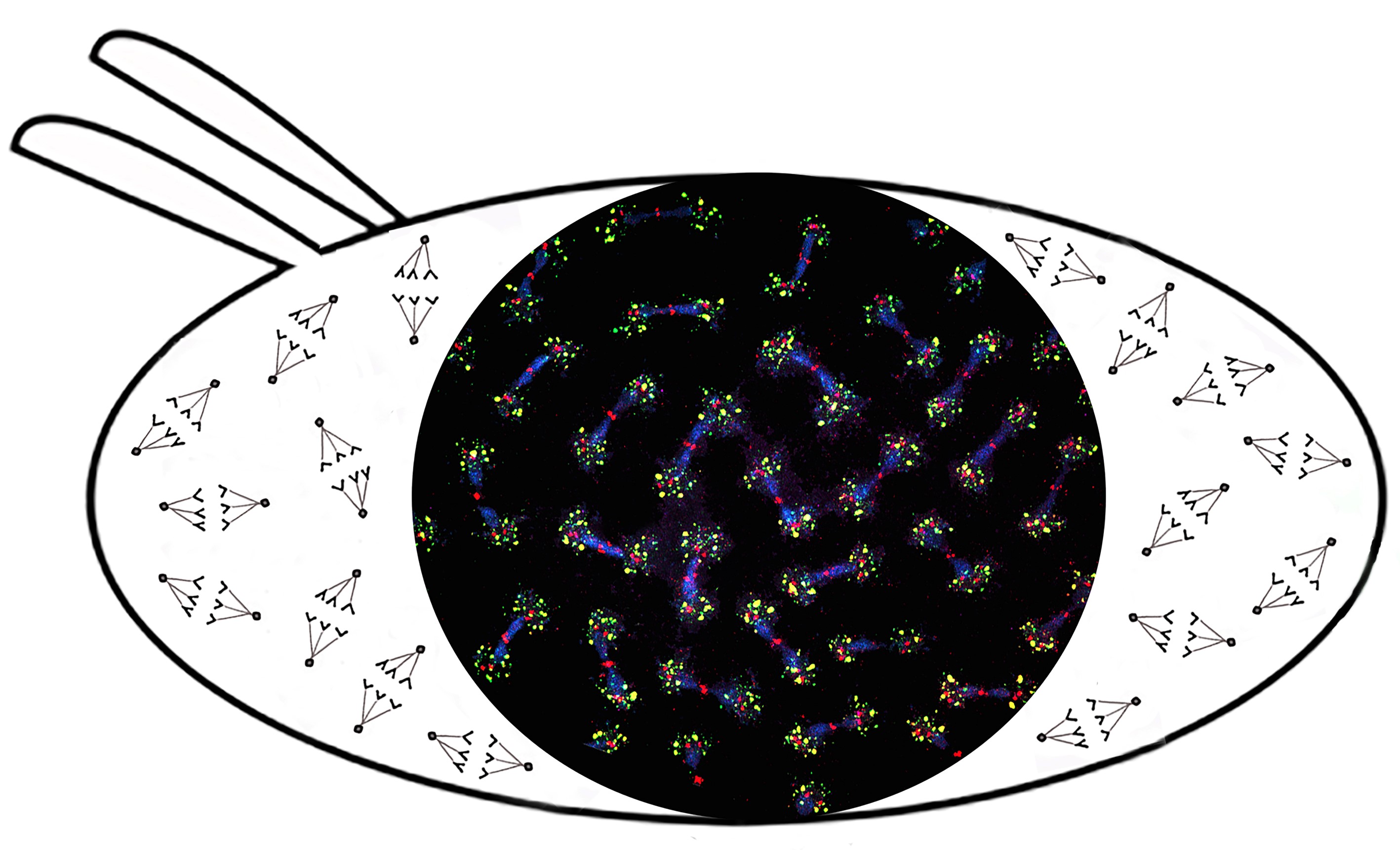
Fig. 4. Aggregation of telomeric RNA (green) with cell cycle kinase Polo (red) around centrosomes leads to disruption of Polo dynamics and mitotic catastrophe in early Drosophila embryogenesis. Mitotic chromosomes in embryonic syncytium after Ars2 knockdown are shown (Morgunova et al. 2021).
During our studies of novel factors involved in telomere maintenance we have identified a new signaling role of telomeric RNAs. We found that when telomeric homeostasis is disturbed, transcription of telomeric repeats is activated, resulting in the accumulation of abundant telomeric ribonucleoprotein complexes (RNPs). The interaction of such telomeric RNPs with key cell cycle proteins causes mitotic defects leading to early developmental arrest (Kordyukova et al. 2018, Morgunova et al. 2021) (Fig. 4). This mechanism ensures the genome stability over generations.
Epigenetics and small RNAs

Fig. 5. piRNAs in the germline can induce the degradation of transposable elements transcripts in the nucleus (left) and the formation of de novo production of piRNAs from transcripts of active copies of transposons (right).
Small RNAs from the class of Piwi-interacting RNAs (piRNA) play a key role in the control of transposable elements in animal gonads. piRNAs in complex with Argonaute proteins of Piwi subfamily interact with the nascent transcripts of transposable elements, which leads to the recruitment of heterochromatin factors to transposons and suppression of their transcription. We have shown that, in addition to transcriptional silencing, piRNAs also attract the Ccr4-Not nuclear deadenylase complex, which leads to co-transcriptional degradation of transposon transcripts, adding another layer of protection against active transposons (Morgunova et al. 2015; Kordyukova et al 2020). We have also discovered a novel and very important ability of piRNAs to induce de novo production of piRNAs at homologous loci of the genome (Fig. 5). This phenomenon was demonstrated for active genomic copies of transposons and explored in detail using a transgenic model (Olovnikov et al. 2013; Shpiz et al. 2014; Akulenko et al. 2018; Komarov et al. 2020). The biological meaning of this phenomenon is the multiplication of piRNAs complementary to active transposons leading to better protection against their expansion and potential genome instability.
We also study the features of the somatic piRNA pathway in Anopheles mosquitoes that can transmit malaria in the wild. In somatic tissues of mosquitoes piRNAs are involved in the antiviral response. Analysis of small RNA libraries from various tissues of mosquitoes shows the presence of new targets of the piRNA-mediated epigenetic regulation.
Regulation of gametogenesis
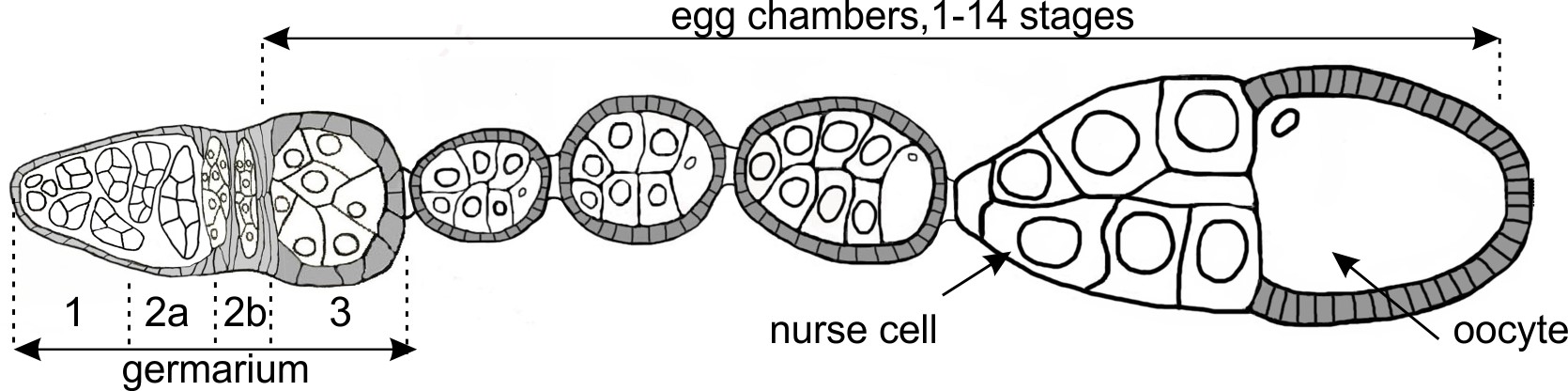
Fig. 6. The main stages of Drosophila melanogaster oogenesis. Scheme of ovariole consisting of germarium and egg chambers at different stages of oogenesis is shown. The germarium is enlarged.
Another area of our research is regulation of gametogenesis. piRNA-mediated control of transposable element activity is an essential factor of normal gametogenesis and fertility (О. Sokolova et al., 2020, Scientific Reports. 10(1):1076; O.A. Sokolova et al. 2019, Mol Biol Cell. 30(12):1544-1554).
Insulator proteins also play an important role in gametogenesis. Their dysfunction leads to sterility and developmental arrest in animals. However, given the multifunctionality of insulator proteins, it is often difficult to identify the mechanism of their action and tissue-specific targets. As a relevant example, null mutants of insulator protein BEAF32 are viable but sterile, which makes it a good model for studying the role of insulators in gametogenesis. We are conducting a genome-wide analysis of chromatin structure and transcriptional changes in order to identify key targets of BEAF32 in Drosophila oogenesis (Fig. 6).
The lab members

Alla Kalmykova
Head of the Lab, PhD, Doctor of Biological Sciences
Research interests: retrotransposons, telomeres, chromatin, small RNAs, development, Drosophila.

Olesya Sokolova
Ph.D., senior researcher
Research interests: regulation of gametogenesis, control of retrotransposon activity, telomeric chromatin, genome-wide methods for analysis of chromatin and transcription, Drosophila.

Anastasia Kobelyatskaya
Ph.D., researcher
Research interests: data analysis, bioinformatics, machine learning, molecular processes of aging, development, molecular oncology.

Valeria Morgunova
Junior researcher
Research interests: gametogenesis, embryogenesis, nuclear topology of telomeres, genetic mechanisms of telomere regulation, confocal microscopy, Drosophila.

Tatyana Sizova
Junior researcher
Research interests: regulation of gene expression, chromatin structure, telomeric RNA, heterochromatin, Drosophila.

Olga Sekreteva
Engineer-researcher
Research interests: Drosophila genetics, genetic crosses and selections, collection maintenance.
Funding
Russian Science Foundation, grant no. 22-14-00006 to A.I. Kalmykova "Mechanisms of telomere stability control in the Drosophila germline".
Russian Science Foundation, grant no. 23-24-00025 to O.A. Sokolova « Genome-wide analysis of the role of insulator complex in transcriptional gene regulation during gametogenesis».
Selected publications:
- Olovnikov I, Ryazansky S, Shpiz S, Lavrov S, Abramov Y, Vaury C, Jensen S, Kalmykova A. De novo piRNA cluster formation in the Drosophila germ line triggered by transgenes containing a transcribed transposon fragment.Nucleic Acids Res. 2013 41: 5757-68, 2013.
- S. Shpiz, S. Ryazansky, I. Olovnikov, Y. Abramov, A Kalmykova. Euchromatic transposon insertions trigger production of novel pi- and endo-siRNAs at the target sites in the Drosophila germline, PLOS Genetics, 10:e1004138, 2014.
- Morgunova V, Akulenko N, Radion E, Olovnikov I, Abramov Y, Olenina LV, Shpiz S, Kopytova DV, Georgieva SG, Kalmykova A. Telomeric repeat silencing in germ cells is essential for early development in Drosophila. Nucleic Acids Res. 43: 8762–8773, 2015.
- E. Radion, S. Ryazansky, N. Akulenko, Y. Rozovsky, D. Kwon, V. Morgunova, I. Olovnikov, A. Kalmykova Telomeric retrotransposon HeT-A contains a bidirectional promoter that initiates divergent transcription of piRNA precursors in Drosophila germline. J. of Mol. Biol. 2017, 429, 3280–3289.
- S. Ryazansky, E. Radion, A Mironova, N Akulenko, Y. Abramov, V. Morgunova, M. Kordyukova, I. Olovnikov, A. Kalmykova. Natural variation of piRNA expression affects immunity to transposable elements. PLoS Genet, 13(4): e1006731, 2017.
- Akulenko, S. Ryazansky, V. Morgunova, P.A. Komarov, I. Olovnikov, C. Vaury, S. Jensen and A. Kalmykova Transcriptional and chromatin changes accompanying de novo formation of transgenic piRNA clusters. RNA, 24: 574-584, 2018.
- M. Kordyukova, I. Olovnikov, A. Kalmykova. Transposon control mechanisms in telomere biology. Current Opinion in Genetics & Development, 49:56-62, 2018.
- E. Radion, V. Morgunova, S. Ryazansky, N. Akulenko, S. Lavrov, Y. Abramov, P. Komarov, S. Glukhov, I. Olovnikov, A. Kalmykova. Key role of piRNAs in telomeric chromatin maintenance and telomere nuclear positioning in Drosophila germline. Epigenetics Chromatin,11(1):40, 2018.
- M. Kordyukova, V. Morgunova, I. Olovnikov, P. A. Komarov, A. Mironova, O. M. Olenkina, A. Kalmykova. Subcellular localization and Egl-mediated transport of telomeric retrotransposon HeT-A ribonucleoprotein particles in the Drosophila germline and early embryogenesis. PLOS One, 13, e0201787, 2018.
- M.Yu. Kordyukova, A.I. Kalmykova. Nature and Functions of Telomeric Transcripts. Biochemistry (Moscow), Vol. 84, No. 2, pp. 137-146, 2019.
- E. Radion, O. Sokolova, S. Ryazansky, P. A. Komarov, Y. Abramov, A. Kalmykova. The Integrity of piRNA Clusters is Abolished by Insulators in the Drosophila Germline. Genes, 10(3), 209, 2019.
- M. Kordyukova, O. Sokolova, V. Morgunova, S. Ryazansky, N. Akulenko, S. Glukhov, A. Kalmykova. Nuclear Ccr4-Not mediates the degradation of telomeric and transposon transcripts at chromatin in the Drosophila germline. Nucleic Acids Res. 48(1):141-156, 2020.
- A. Komarov, O. Sokolova,N. Akulenko, E. Brasset, S. Jensen* and A. Kalmykova Epigenetic Requirements for Triggering Heterochromatinization and Piwi-Interacting RNA Production from Transgenes in the Drosophila Germline. Cells 2020, 9(4), 922.
- V. Morgunova, M. Kordyukova, E.A. Mikhaleva, I. Butenko, O.V. Pobeguts, and A. Kalmykova. Loss of telomere silencing is accompanied by dysfunction of Polo kinase and centrosomes during Drosophila oogenesis and early development. PlosOne, 16(10): e0258156, 2021.
- V. V. Morgunova, O. A. Sokolova, T.V. Sizova, L.G. Malaev, D.S. Babaev, D.A. Kwon, and A. I. Kalmykova, Dysfunction of Lamin B and Physiological Aging Cause Telomere Instability in Drosophila Germline. Biochemistry (Moscow), Vol. 87, Nos. 12-13, 2022.
