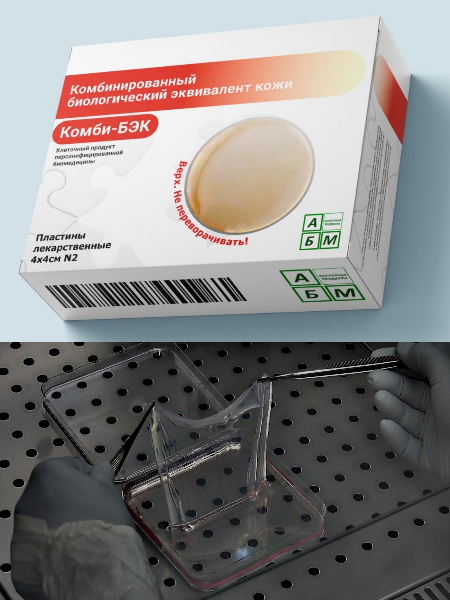
On 11 October 2024, the permission of the Ministry of Health of the Russian Federation was obtained to conduct clinical trials of a medicinal product (tissue engineering product) developed at IDB RAS, "Combined Biological Skin Equivalent (CombiBSE)" to assess safety and efficacy in the treatment of patients with heat injuries. The unique feature of the development of CombiBSE is the combination of donor mesenchymal stem cells and autologous keratinocytes of the patient in one product.
The research was conducted on the base of Government basic research program No. 0088-2023-0001 Theme "Development of biomedical cell products and creation of a pilot bank of tissue and cell transplants".
This clinical trial of a high-tech drug based on cultured human cells is the second in the Russian Federation.
Akrus Biomed Ltd. is an industrial partner of IDB RAS for the future clinical trials. Its novel industrial platform will become a place to produce samples of CombiBSE, which later will be proceed to multicenter randomized open-label clinical trials.