Microscopy & Microdissection
Confocal Microscopy
Leica TCS SP5
Confocal Laser Scanning Microscope

Lasers: 488 nm, 561 nm, 590 nm, 633 nm.
4 PMT detectors, Transmitted light detector.
Fluorescent microscopy, Nomarsky (Differential interference contrast microscopy, DIC), FRET/FRAP.
CO2-incubator.
Fluorescence recovery after photobleaching (FRAP) is a method for determining the kinetics of diffusion through tissue or cells. This technique is very useful in biological studies of cell membrane diffusion and protein binding. https://en.wikipedia.org/wiki/Fluorescence_recovery_after_photobleaching
Förster or fluorescence resonance energy transfer (FRET), resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). Measurements of FRET efficiency can be used to determine if two fluorophores are within a certain distance of each other. Such measurements are used as a research tool in fields including biology and chemistry. https://en.wikipedia.org/wiki/F%C3%B6rster_resonance_energy_transfer
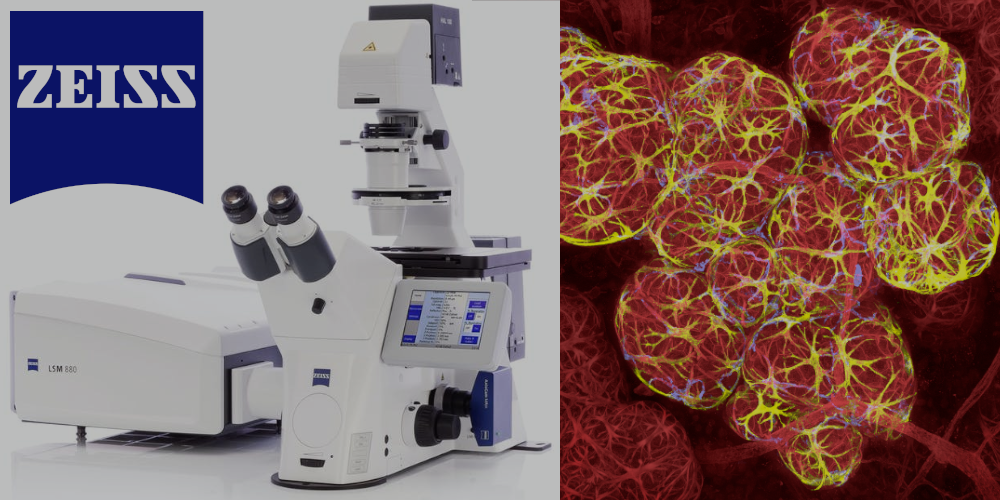
Zeiss LSM880
Confocal Laser Scanning Microscope with Airyscan and FLIM modules.


Lasers: 405 nm, 440 nm (FLIM-module), argon lasers (458 nm, 488 nm, 514 nm), 543 nm, 633 nm.
Objectives: 5x/0,16, 10x/0,45, 20x/0,8, 40х/1,4 (oil), 63х/1,4 (oil).
Detectors: 2 PMT detectors, highly-sensitive GaSaAP detector, transmitted light detector (T-PMT).
Fluorescent microscopy, Airyscan, Nomarsky (Differential interference contrast microscopy, DIC), FRET/FRAP, FLIM,
CO2-incubator.
"With Airyscan, ZEISS introduced a new detector concept for confocal laser-scanning microscopy (LSM). Whereas traditional LSM designs use a combination of pinhole and single-point detectors, Airyscan is a 32-channel gallium arsenide phosphide photomultiplier tube (GaAsP-PMT) area detector that collects a pinhole-plane image at every scan position. Each detector element functions as a single, very small pinhole. Knowledge about the beam path and the spatial distribution of each detector channel enables very light-efficient imaging with improved resolution and signal-to-noise ratio" (Huff, 2015). https://doi.org/10.1038/nmeth.f.388.
"Fluorescence-lifetime imaging microscopy (FLIM) - is an imaging technique based on the differences in the exponential decay rate of the photon emission of a fluorophore from a sample. This technique has the advantage of minimizing the effect of photon scattering in thick layers of sample. Being dependent on the micro-environment, lifetime measurements have been used as an indicator for pH, viscosity and chemical species concentration" https://en.wikipedia.org/wiki/Fluorescence-lifetime_imaging_microscopy.
Light / Fluorescence Microscopy
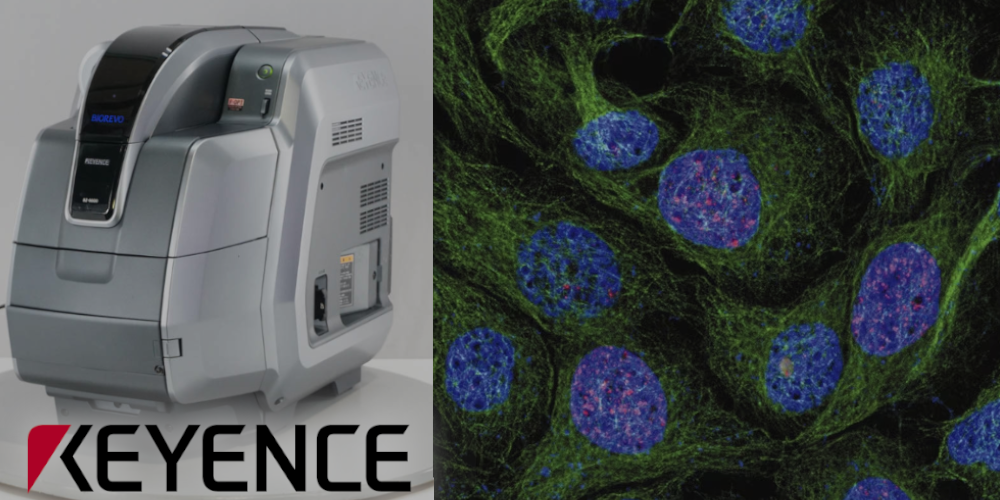
Keyence BZ-9000E
Inverted fluorescence phase-contrast microscope "All-in-One"

Filer Cubes: DAPI, GFP, Texas-R, Cy5
Objectives: 4x/0.20, 20x/0.45 (PlanFluor), 20х/0.75, 40x/0.95, 60x/1.40 (oil), 100x/1.40 (oil)
Image pick-up: 2/3 inch, 1.5 million pixel monochrome CCD (colorized with LC filter)
Bright field, fluorescence, phase contrast
CO2-incubator
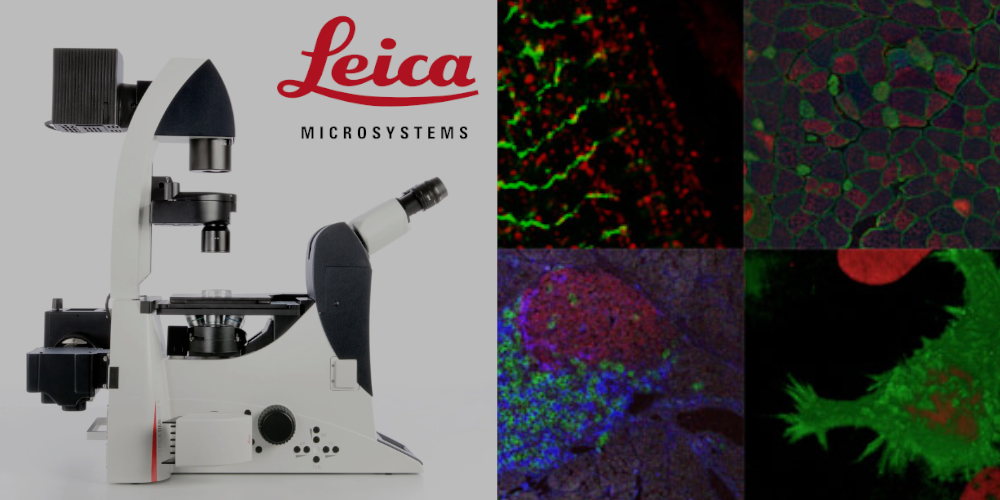
Leica DMI6000
Inverted fluorescence microscope.

Lasers: 365 nm, 470 nm, 530 nm, 590 nm, 625 nm
Objectives: 10x/0.3, 20x/0.4, 20x/0.7 (immersion), 40x/0.8, 63x/0.7, 100x/1.4 (oil)
Image pick-up: Leica DFC 360 FX CCD, b/w, 2/3 inch, 1.4 МP (1392 x 1040 pixels).
Fluorescent microscopy (samples can be imaged in vivo), Nomarsky (Differential interference contrast microscopy, DIC), visualization of double photon excitation (Fluo), FRET.
Laser Microdissection (LMD) System
Leica LMD7000

LMD laser' characteristics:
- Wavelength 349 nm
- Pulse frequency 10–5.000 Hz
- Pulse length < 4 ns
- Max. pulse energy 120 µJ
- Max. area od dissection - 2 mm2
Lasers for Fluorescent Microscopy: LMD UV; blue;green 420/30 495/15 570/200
Objectives: 5x/0,12, 10x/0,3, 20x/0,4, 40х/0,6, 63x/0,70, 150х/0,90 (oil).
Digital camera: Hitachi HV-D20P (CCD, 1/2 inch, 800 ТВЛ)
Bright field, fluorescent microscopy, polarized light microscopy, Nomarsky (Differential interference contrast microscopy, DIC).
Leica LMD uses a microscope to visualize individual cells or cell clusters. The Leica LMD perform sample preparation for molecular biology analysis directly from the tissue section. Regions of interest are selected by a software, excised from the surrounding tissue by a UV laser, and collected easily and safely by gravity into vessels.
Zeiss AxioImager M1 with a Colibri LED light source
The Zeiss Axio Imager M1 is a compound, upright, high-performance microscope for imaging samples on slides. The system is capable of brightfield, DIC (Differential Interference Contrast), and fluorescence imaging using a Colibri 7 LED configurable light source. The fully motorised stage and filter turret is controlled using ZEN software and allows multi-dimensional data acquisition, such as multiple fluorescence channels, tiled images, Z-stacks, and time lapse. Multicolor LED Light Source for Gentle Fluorescence Imaging
Operating Manual Axio Imager Upright Microscope
Colibri 7 LED light source
Line / Wavelength / Bandwidth:
UV / 385 / 30 nm
V / 423 / 44 nm
B / 469 / 38 nm
C / 511 / 44 nm
Nexcope NSZ-818M
"Nexcope" (http://www.nexcope.com) is a research-grade microscope brand registered by Ningbo Yongxin Optics Co., Ltd.
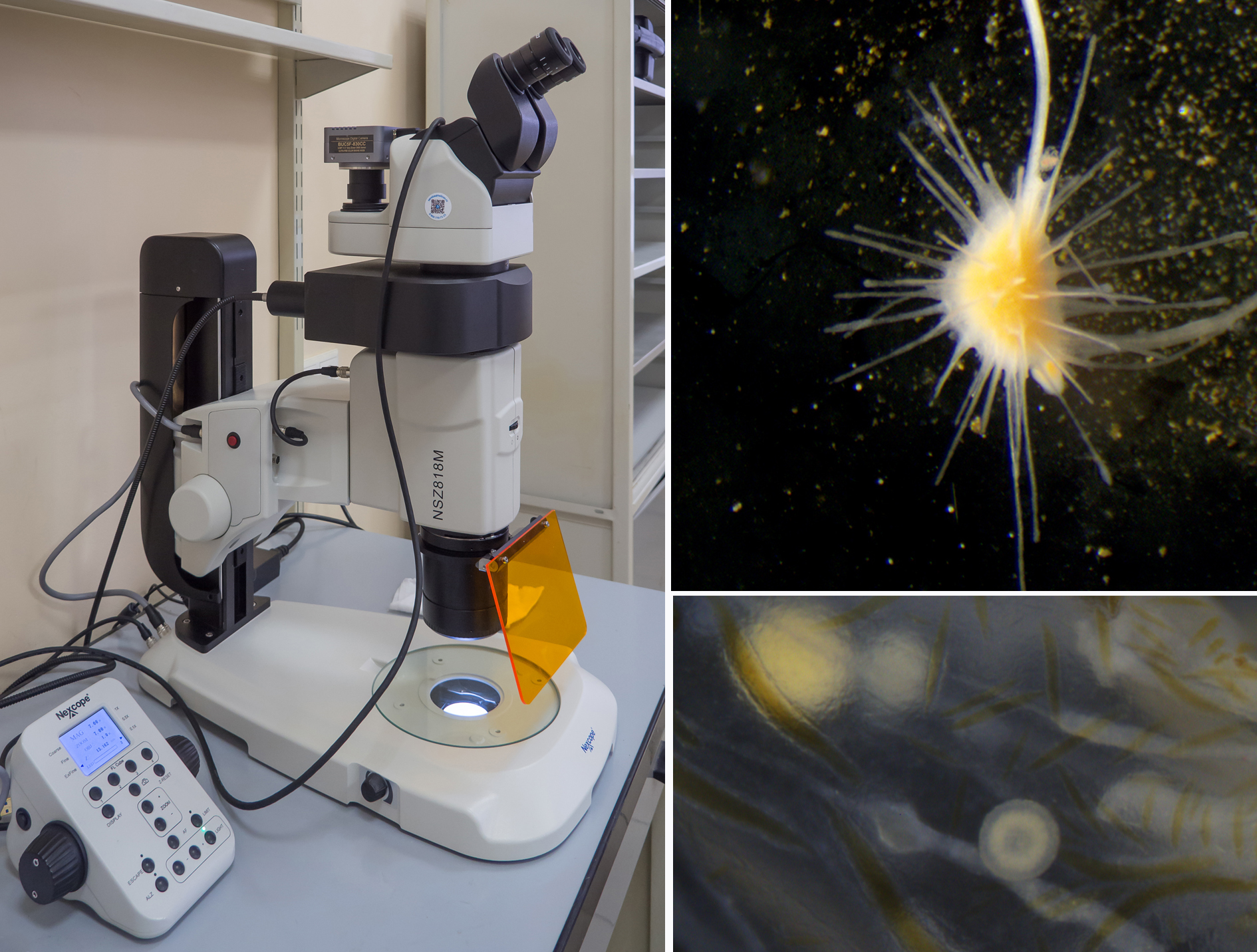
The Nexcope NSZ-818M is a motorized stereo microscope with an epi fluorescent module and a digital camera. The microscope includes: a high-performance plan-apochromatic lens with a working distance of 60 mm, adjustable diaphragm, the 10x wide-field eyepieces with a large 22 mm field of view, the dioptre adjustment on both sides, LED transmitted-light illuminator, the focusing system that can be used in the coarse, fine and extra fine modes for sensitive focusing, even at high magnifications.
Visual system:
- Trinocular tube with variable tilt angle 0-30°, interpupillary distance 50-76 mm. (100:0 / 0:100)
- Eyepieces - 10x(22 mm field of view)
- In the photo mode, the beam path for the right eye is redirected to the camera
- Transfocator - 18:01
- Magnification range - 0.75x - 13.5x
- Aperture diaphragm - Built-in
- Lens Plan APO - 1x, NA 0.15, working distance 60 mm
Focusing system:
- Coaxial coarse, fine and extra fine adjustment,
- Focusing accuracy 2 µm,
- Range of movement - 60 mm.
Stand base & Illumination:
- Stand base - 365 x 314 mm, with built-in illuminator
- Diascopic illumination system - 5W LED, oblique coherent illumination system
- Epi fluorescent module - Turret for mounting filter cubes
- Fluorescent illuminators - External LED, 4-channel LED
- Epi fluorescence filter sets - B / G / U / DAPI / FITC / TRITC / R
Leica DMi8 inverted microscope
Leica DMi8 premium inverted research microscope providing fluorescence observation, phase contrast, light field.
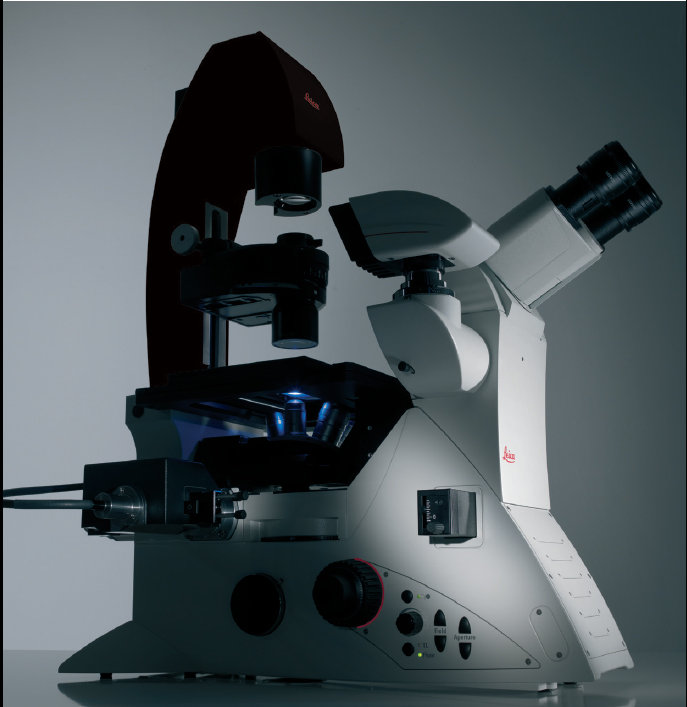
The microscope has 6-position objective turret with 5 x, 10 x, 20 x LWD, 20x, 40x (aqueous), 40x LWD, 63x (oil) objectives. Trinocular tube, field of view is 25 mm, eyepieces 10x / 25 mm.
Leica DMi8 has a set of excitation wavelengths: 395, 475, 555, 635nm. The power of each individual light source not less than 200mV, with the ability to independently adjust the intensity of each wavelength.
Leica DMi8 is equipped with the motorized turret of four-band fluorescence light filter system, 2 universal filter systems and a filter set for a fast filter wheel.
Fixed stage of the microscope has a range of movement of 120 mm along the X axis and 80 mm along the Y axis. There are several sample holders: universal, for slides and Petri dishes of different diameters, and for multi-well plates.
The THUNDER Technology
THUNDER is an opto-digital technology that uses the Computational Clearing method to generate high resolution and high contrast images. Computational Clearing removes the typical haze inherent to all widefield images of thick samples. It produces brilliant results for large image stacks, as well as single images taken deep in your sample.
THUNDER, a Leica technology, automatically takes all relevant optical parameters into account in order to achieve haze-free results in real time.